Copper sulfate ia a required material for the preparation of TACC (Section I, No. 16).
MATERIAL REQUIRED:
- Pieces of copper or copper wire
- Dilute sulfuric acid (battery acid)
- Potassium Nitrate (Section I, No. 2) or
- Nitric Acid, 90%conc. (1.48 sp. gr.) (Section I, No. 4)
- Alcohol Water
- Two 1 pint jars or glasses, heat resistant
- Paper towels
- Pan
- Wooden rod or stick
- Improvised Scale (Section VII, No. 8)
- Cup
- Container Heat source Teaspoon
PROCEDURE:
1. Place 10 grams of copper pieces Lnto one of the pint jars. Add 1 cup (240 milliliters) of dilute sulfuric acid to the copper.
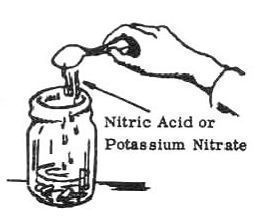
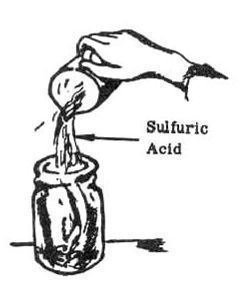 2. Add 12 grams of potassium nitrate or 1-1/2 teaspoons of nitric acid to the mixture.
2. Add 12 grams of potassium nitrate or 1-1/2 teaspoons of nitric acid to the mixture.
NOTE: Nitric acid gives a product of greater purity.
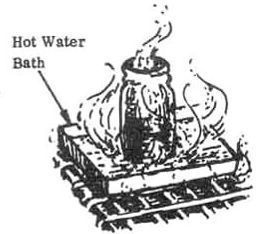
3. Heat the mixture in a pan of simmering hot water bath until the bubbling has ceased (approximately 2 hours). The mixture will turn to a blue color.
CAUTION: The above procedure will cause strong toxic fumes. Perform Step 3 in an open, well ventilated area.
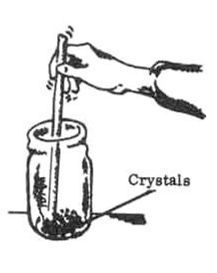
4. Pour the hot blue solution, but not the copper, into the other pint Jar. Allow solution to cool at room temperature. Crystals will form at the bottom of the Jar. Discard the unreacted copper pieces in the first Jar.
5. Carefully pour away the liquid from the crystals. Crush crystals Into a powder with wooden rod or stick.
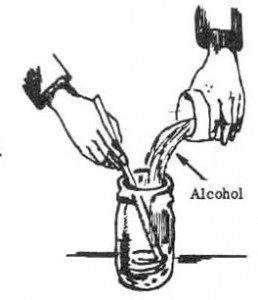
6. Add 1/2 cup (120 milliliters) of alcohol to the powder while stirring.
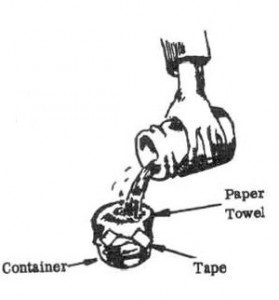
7. Filter the solution through a paper towel into a container to collect the crystals. Wash the crystals left on the paper towel three times, using 1/2 cup (120 milliliters) por-tions of alcohol each time.
9. Air dry the copper sulfate crystals for 2 hours.
NOTE: Drying time can be reduced to 1/2 hour by use of hot, not boiling, water bath (see Step 3).
Preparation of Copper Sulfate (Pentahydrate),