Potassium or sodium nitrite is needed to prepare DDNP (Section I, No. 19), and litharge is required for the preparation of lead picrate (Section I, No. 20).
MATERIAL REQUIRED:
- Lead metal ismall pieces or chips)
- Potassium (or sodium) nitrate
- Methyl (wood) alcohol
- Iron pipe with end cap
- Iron rod or screwdriver
- Paper towels
- 2 glass jars, wide mouth
- Metal pan
- Heat source (hot coals or blow torch)
- Improvised scale (Section VII, No.
- Cup
- Water
- Pan
SOURCE:
- Plumbing supply store
- Field grade (Section I, No. 2) or
- Drug Store
PROCEDURE:
1. Mix 12 grams of lead and 4 grams of potassium or sodium nitrate in a jar. Place the mixture in the iron pipe.
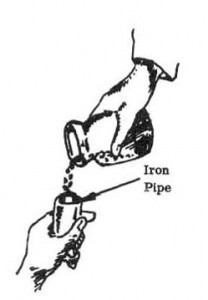 2. Heat iron pipe in a bed of hot coals or with blow torch for 30 minutes to 1 hour. (Mixture will change to a yellow color.)
2. Heat iron pipe in a bed of hot coals or with blow torch for 30 minutes to 1 hour. (Mixture will change to a yellow color.)
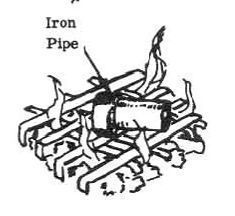 3. Remove the iron pipe from the heat source and allow to cool. Chip out the yellow material formed in the Iron pipe and place the chips in the glass jar.
3. Remove the iron pipe from the heat source and allow to cool. Chip out the yellow material formed in the Iron pipe and place the chips in the glass jar.
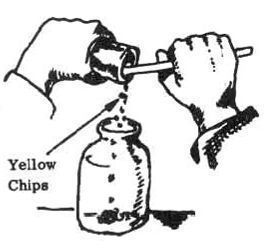 4. Add 1/2 cup (120 milliliters) of methyl alcohol to the chips.
4. Add 1/2 cup (120 milliliters) of methyl alcohol to the chips.
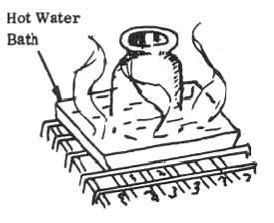
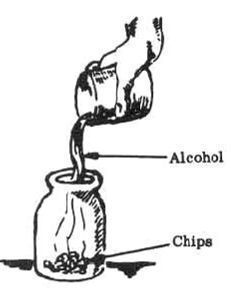 5. Heat the glass jar containing the mixture in a hot water bath for approximately 2 minutes (heat until there is a noticeable reaction between chips and alcohol; solution will turn darker).
5. Heat the glass jar containing the mixture in a hot water bath for approximately 2 minutes (heat until there is a noticeable reaction between chips and alcohol; solution will turn darker).
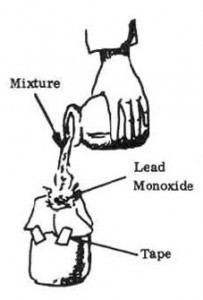
6. Filter the mixture through a paper towel into the other glass jar. The material left on the paper towel is lead monoxide.
7. Remove the lead monoxide and wash it twice through a paper towel using 1/2 cup (120 milliliters) of hot water each time. Air dry before using.
8. Place the Jar with the liquid (from Step 5) in a hot water bath (as in Step 5) and heat until the alcohol has evaporated. The powder remaining in the Jar after evaporation is potassium or sodium nitrite.
NOTE: Nitrite has a strong tendency to absorb water from the atmosphere and should be stored in a closed container.