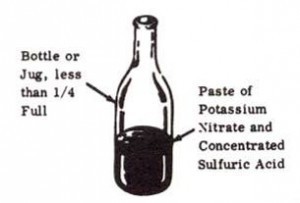
Nitric acid is used in the preparation of many explosives, incendiary mixtures, and acid delay timers. It may be prepared by distilling a mixture of potassium nitrate and concentrated sulfuric acid.
MATERIAL REQUIRED:
- Potassium nitrate (2 parts by volume)
- Concentrated sulfuric acid (1 part by volume)
- 2 bottles or ceramic jugs (narrow necks are preferable)
- Pot or frying pan
- Heat source (wood, coal, or char¬coal)
- Tape (paper, electrical, masking, etc. but not cellophane)
- Paper or raga
SOURCES:
- Drug Store
- Improvised (Section I, No. 2)
- Motor vehicle batteries
- Industrial plants
IMPORTANT: If sulfuric acid is obtained from a motor vehicle batten*. concentrate it by boiling It until white fumes appear. DO NOT INHALE FUMES.
NOTE: The amount of nitric acid produced is the same as the amount of potassium nitrate. Thus, for 2 tablespoonsful of nitric acid, use 2 tablespoonsful of potassium nitrate and 1 tablespoonsful of concentrated sulfuric acid.
PROCEDURE:
1. Place dry potassium nitrate in bottle or jug. Add sulfuric acid. Do not fill bottle more than 1/4 full. Mix until paste is formed.
CAUTION: Sulfuric acid will burn skin and destroy clothing. If any is spilled, wash it away with a large quantity of water. Fumes are also dangerous and should not be inhaled.
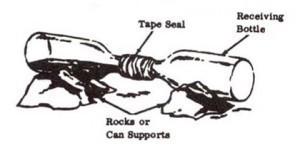
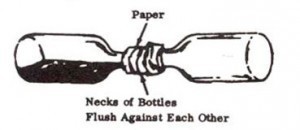
2. Wrap paper or rags around necks of 2 bottles. Securely tape necks of bottles together. Be sure bottles are flush against each other and that there are no air spaces.
 3. Support bottles on rocks or cans so thit empty bottle Is slightly lower than bottle containing paste so that nitric acid that is formed in receiving bottle will not run into other bottle.
3. Support bottles on rocks or cans so thit empty bottle Is slightly lower than bottle containing paste so that nitric acid that is formed in receiving bottle will not run into other bottle.
4. Build fire in pot or frying pan.
5. Gently heat bottle containing mixture by moving fire in and out. As red fumes begin to appear periodically pour cool water over empty receiving bottle. Nitric acid will begin to form In the receiving bottle.
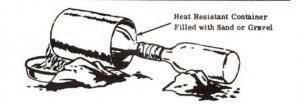
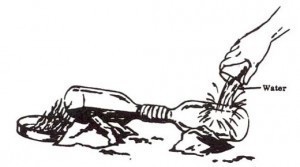 CAUTION: Do not overheat or wet bottle containing mixture or it may shatter. As an added precaution, place bottle to be heated in heat resistant container filled with sand or gravel. Heat this outer container to produce nitric acid.
CAUTION: Do not overheat or wet bottle containing mixture or it may shatter. As an added precaution, place bottle to be heated in heat resistant container filled with sand or gravel. Heat this outer container to produce nitric acid.
6. Continue the above process until no more red fumes are formed. If the nitric acid formed in the receiving bottle is not clear (cloudy) pour it into cleaned bottle and repeat Steps 2-6.
CAUTION: Nitric acid will burn skin and destroy clothing. If any is spilled, wash it away with a large quantity of water. Fumes are also dangerous and should not be inhaled.
Nitric acid should be kept away from all combustibles and should be kept in a sealed ceramic or glass container.